Chapter 5 Electrons In Atoms Answer Key
One CC σ bond results from overlap of sp 2 hybrid orbitals on the carbon atom with one sp 2 hybrid orbital on the other carbon atom. Radioactive decay also known as nuclear decay radioactivity radioactive disintegration or nuclear disintegration is the process by which an unstable atomic nucleus loses energy by radiationA material containing unstable nuclei is considered radioactiveThree of the most common types of decay are alpha decay α-decay beta decay β-decay and gamma decay γ.

Chapter 5 Electrons In The Atom Ppt Download
The 2p y and 2p z electrons of the carbon atoms now form pi bonds with each other as illustrated below.
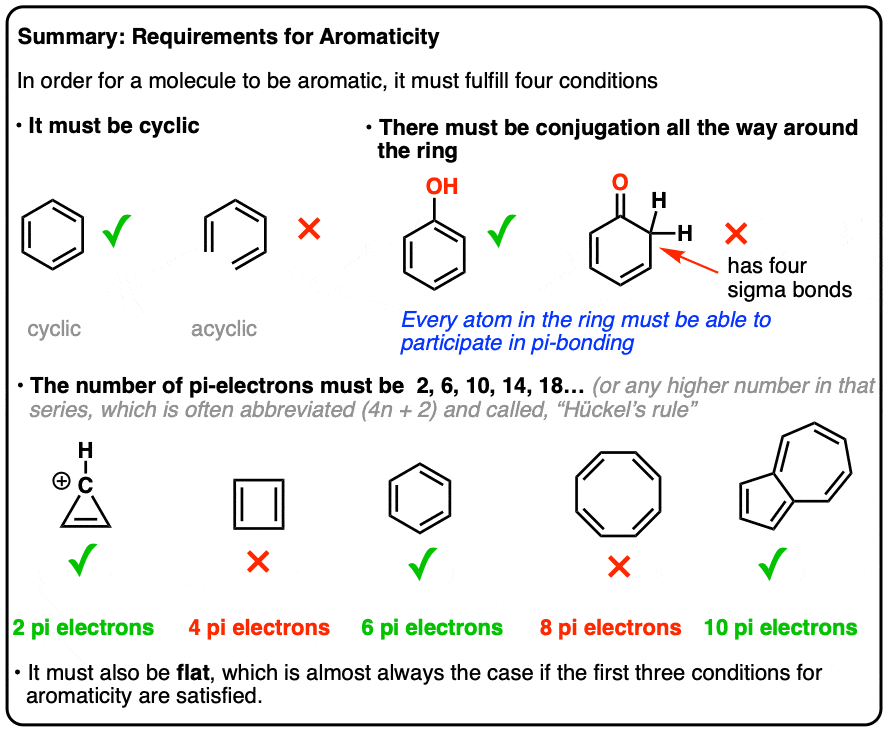
. The bond which is formed by the transfer of electrons between the atoms is called electrovalent bond or ionic bond. There are three isotopes of hydrogen namely protium 1 1 H deuterium 2 1 H or D and lastly tritium 3 1 H or T. Unit 8 has a good weightage in entrance exams like JEE and NEET.
The nitrogen in C 5 H 5 N would act as a proton acceptor and therefore can be considered a base but because it does not contain an OH compound it cannot be considered a strong base. Those elements which have the same atomic number but a different mass number are called isotopes. ACG 2021 Quiz.
These intermolecular forces occur between non-polar substances. 232 Digestive System Processes and Regulation. Final Paper - COM 315.
The sp hybridized orbital in the carbon atom can form a total of two sigma bonds. In a reaction 53g of sodium carbonate reacted with 6 g of acetic acid. In order to understand the.
This lesson is intended as a follow-up to chapter 4 lesson 2. 237 Chemical Digestion and Absorption. The NCERT Solutions for Class 12 Physics Chapter 6 PDF is provided here to help students understand the chapter in an easy and interesting way.
M ost systems or processes depend at some level on physical and chemical subprocesses that occur within it whether the system in question is a star Earths atmosphere a river a bicycle the human brain or a living cell. BANA - Chapter 51 Notes. The difference in the mass of 100 moles each of sodium atoms and sodium ions is 548002 g.
BANA 2082 - Quiz 73 WebAssign. B The π bond is formed by the side-by-side overlap of the two. For Lesson 43 students can.
Both electrons and holes are equal in magnitude but opposite in polarity. 2n 2 where n 1 2 3 Maximum number of electrons in different. Atoms may be depicted by circle shapes each of which has a nucleus at the center containing protons and neutrons surrounded by one or more concentric circles representing the shells or levels in which the electrons surrounding the nucleus of the atom are located and markings indicating the electronat each level.
Because HCl is listed in Table 121 it is a strong acid. Electrovalent bonds are produced when electrons are transferred from atoms of one element to atoms of another element producing positive and negative ions. NCERT Solutions for Class 11 Chemistry Chapter 5 States of Matter are available on this page to help students get a clear idea of the basic concepts.
NCERT Solutions for Class 12 Physics Chapter 6 Free PDF Download. Four CH bonds result from the overlap between the C atoms sp 2 orbitals with s orbitals on the hydrogen atoms. Access Answers of Science NCERT Class 9 Chapter 3 Atoms and Molecules All in text and Exercise Questions solved Class 9 Science Chapter 3 Exercise-31 Questions with Answer Exercise-31 Page.
233 The Mouth Pharynx and Esophagus. Sodium atom and ion differ by one electron. Who Killed Barry mystery game find out who killed barry.
Compute the mass of an electron. From the Bohr-bury scheme we can say that the outermost shell can contain a maximum of 8 electrons. Chemistry is simply numbers an idea Pythagoras would have liked.
Due to these forces they can condense to liquids and or freeze into solids at low temperature. Dimension 3 DISCIPLINARY CORE IDEASPHYSICAL SCIENCES. To illustrate an atom of an alkali metal group 1 loses one electron and forms a cation with a 1 charge.
231 Overview of the Digestive System. Figure 823 In the ethene molecule C 2 H 4 there are a five σ bonds. The chemistry of an atom depends only on the number of electrons which equals the number of protons and is called the atomic number.
The electrons in the energy level farthest from the nucleus are called valence electrons. 235 The Small and Large Intestines. Detailed and step-by-step solutions.
Large-scale systems often have emergent properties that cannot be explained on the basis of. The atom is the most fundamental unit of matter making up everything that we see around us. 236 Accessory Organs in Digestion.
As per the latest CBSE syllabus Unit-7 Dual Nature of Radiation Matter Unit-8 combined has a weightage of 12 marks in the board exam. A Closer Look. Some key characteristics of a valence electron are.
Rutherfords Model of Atoms. Atoms in the same column group in the periodic table have the same number of valence electrons. Atoms transfer or share electrons in such a way that they can attain a filled shell of electrons.
The Higgs boson sometimes called the Higgs particle is an elementary particle in the Standard Model of particle physics produced by the quantum excitation of the Higgs field one of the fields in particle physics theory. For the main group elements the valence electron exists only in the outermost electron shell. Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
The NCERT Solutions for Class 12 Physics Chapter 6 Electromagnetic Induction is crucial for the students of 12 th standard. BANA 2082 - Exam 1 study guide part 4. Hydrogen is the first element in the periodic table and has the atomic number one.
Holes and electrons are the types of charge carriers accountable for the flow of current in semiconductors. Ati nursing care of children rn 2019 proctored exam. It is extremely small measuring in at less than 01 to 05 nanometers.
For Chapter Summary On Atoms And Molecules Watch The Below Video. The isotopes are different because of the different number of neutrons. An alkaline earth metal group 2 loses two electrons and forms a cation with a 2 charge and so on.
Students may wonder why an energy level can hold only a. Mobility of Electrons and Holes. For 100 moles each of sodium atoms and ions there would be a difference of 100 moles of electrons.
Electrovalent bonds are only formed between metals and non-metals. Bohrs model of the atom. 234 The Stomach.
One sigma bond is formed with the adjacent carbon atom and the other is formed with the 1s orbital belonging to the hydrogen atom. Straighterline A. C 5 H 5 N.
Atoms are most stable if they have a filled valence shell of electrons. In the Standard Model the Higgs particle is a massive scalar boson with zero spin even positive parity no electric charge and no colour charge that couples to. 10 WEEK MENS Program Final.
The Liver Pancreas and Gallbladder. It is a weak base. When the electrons in two adjacent atoms are displaced in such a way that atoms get some temporary dipoles they attract each other through the London dispersion force.
NCERT Solutions Class 11 Chemistry Chapter 5 Free PDF Download According to the latest update on the CBSE Syllabus 2022-23 this chapter has been removed. We all know how electrons in an atom are arranged in shellsorbitals. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas.
Mass of 100 moles of electrons 548002 g. Holes valence electrons are the positively charged electric charge carrier whereas electrons are the negatively charged particles. Maximum number of electrons that can be accommodated in a shell is given by the formula.
Two helium Carl Sagan Cosmos 1980 Random House p223. Class 12 Physics Chapter 12 Atoms comes under Unit-8 Atoms and Nuclei. Because MgOH 2 is listed in Table 121 it is a strong base.
Atoms and molecules are responsible for forming tiny sand particles gargantuan black holes and everything in between. If you are an atom with one proton you are hydrogen. Only a little chemical activity is observed when the outermost shell is completely.
Valence electrons are those electrons which are present in the outermost orbit of the atom.

Chapter 5 Worksheets Pdf
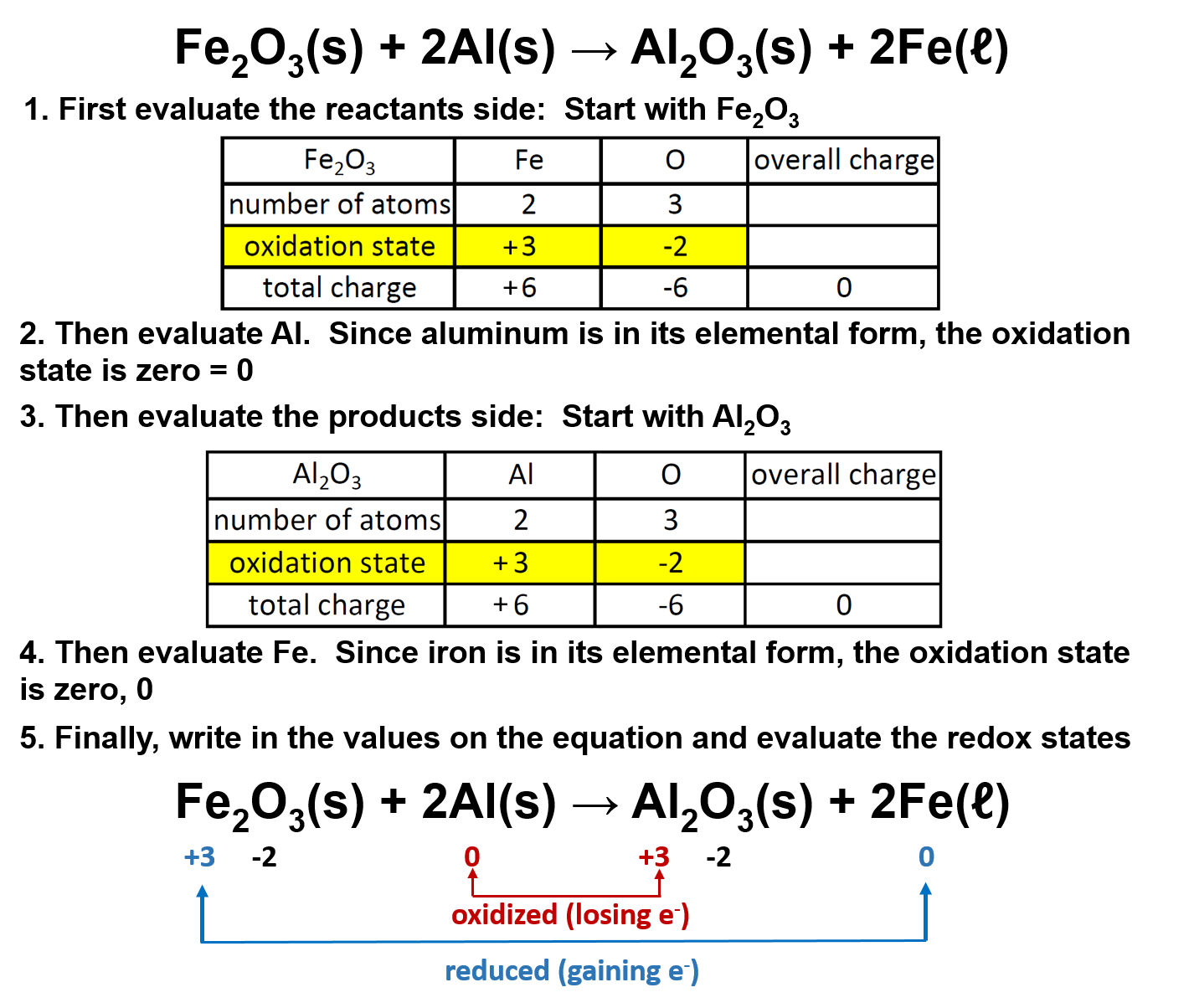
Ch104 Chapter 5 Chemical Reactions Chemistry

Oswaal Ncert Problems Solutions Textbook Exemplar Class 9 Science Book For 2022 Exam Oswaal Books Learning Pvt Ltd Amazon In Books

20 Solved Questions In Principles Of Chemistry I Quiz 2 Ch 301 Quizzes Chemistry Docsity

Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry
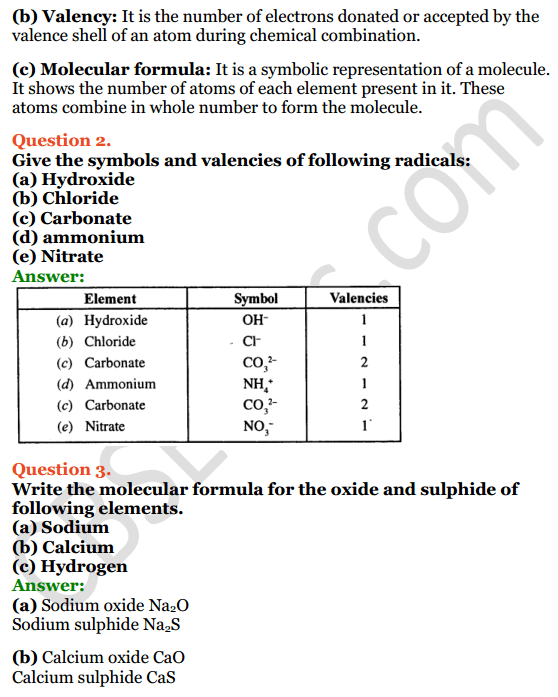
Selina Concise Chemistry Class 8 Icse Solutions Chapter 5 Language Of Chemistry Cbse Tuts
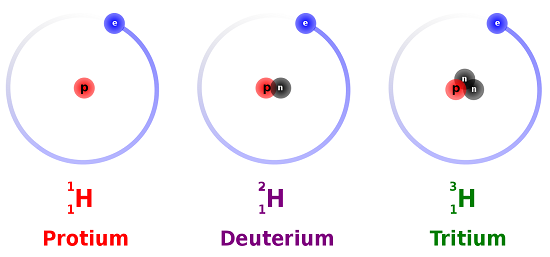
4 8 Isotopes When The Number Of Neutrons Varies Chemistry Libretexts
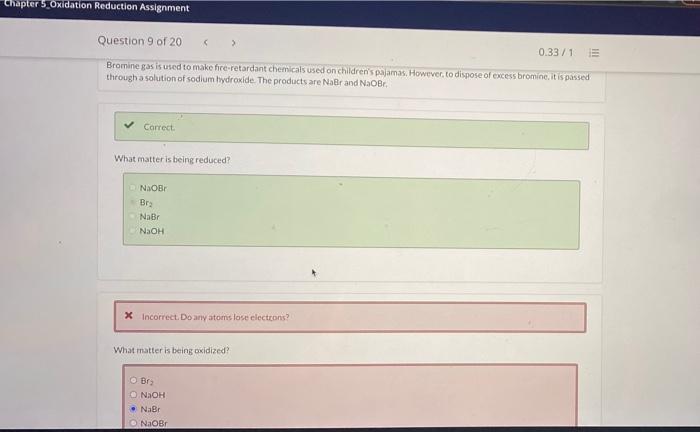
Solved Chapter 5 Oxidation Reduction Assignment Question 9 Chegg Com

Atoms Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Kesler Science
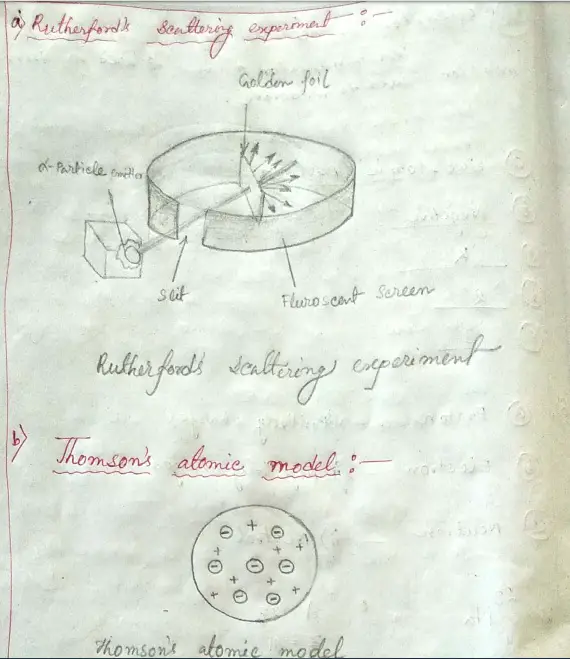
Maharashtra Board Class 8 Science Chapter 5 Inside The Atom Solution

Two Elements P And Q Belong To The Same Period Of The Modern Periodic Table And Are In Group 1 And G

Class 10 Science Page 2 Class 10 Science

Name Date Class Electrons In Atoms Standard Curriculum Core Content Extension Topics Pdf Free Download

Chemistry 105 Problem Set 09 Electron Configurations Problem Set 09 Electron Configurations Studocu
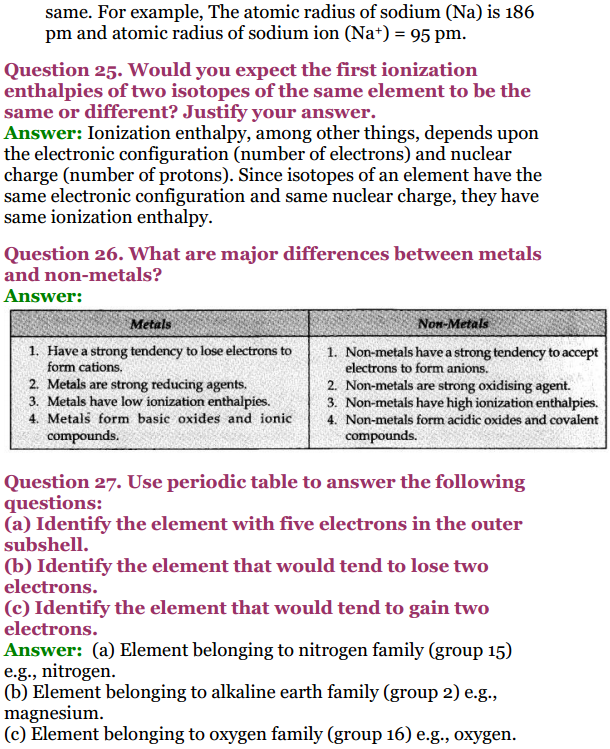
Ncert Solutions For Class 11 Chemistry Updated For 2020 21

Selina Solutions Class 9 Concise Chemistry Chapter 5 The Periodic Table Download Free Pdf

Cpo Chapter 14 Physical Science Barry Vetter Blackman High School